The goal of the vaccine-approval process is to end up with a vaccine that is effective (the vaccine works in preventing the illness) and safe (there are no serious side effects or other problems). In the United States, this process has produced safe and effective vaccines for the flu, polio, measles, mumps, pertussis, and more. Over time, this process has saved millions of people from getting sick and dying.
The stages of development generally follow this timeline:
- Exploratory stage: This is the start of lab research to find something that can treat or prevent a disease. Vaccine development typically begins not at a pharmaceutical company, but in a research laboratory in a university, medical center, or biotech company. Scientists in these laboratories are most often funded by grants from the government or private foundations.
- Pre-clinical stage: Scientists use lab tests and testing in animals, such as mice or monkeys, to learn whether a vaccine might work. Many potential vaccines do not make it past this point. If the tests are successful, and the U.S. Food and Drug Administration (FDA) signs off, it’s on to clinical testing.
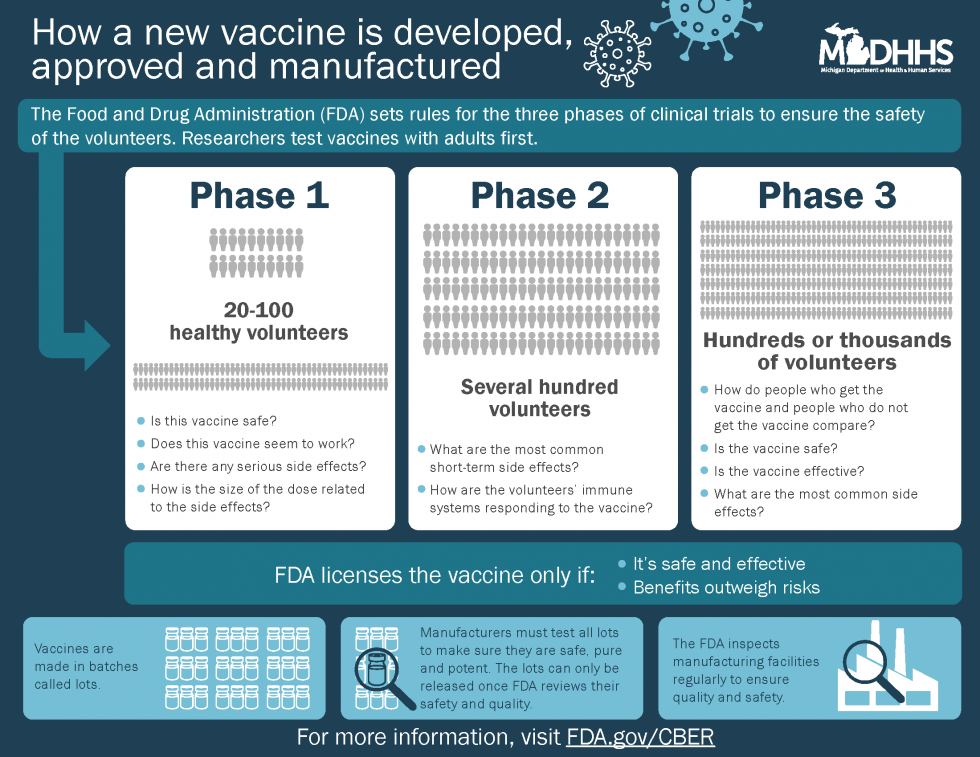
- Clinical development: This is a three-phase process of testing in humans.
- Phase I usually involves fewer than 100 people and seeks to answer two main questions: does the vaccine generate the expected immune response (does it work in creating antibodies to protect someone from the disease) and is the vaccine safe (does the vaccine show any serious side effects)?
- Phase II involves several hundred people, comparing those who did and did not receive vaccine. During this phase, scientists try to determine the proper dose of vaccine to be given, and they continue to study the vaccine’s safety. They also determine how to manufacture the vaccine — making sure the process and packaging creates a consistent vaccine, so that each batch produces similar results.
- Phase III involves tens of thousands of study participants who are similar to the population that will receive the vaccine, again comparing those who did and did not receive vaccine. During these studies, as with the previous phases, no one working with the patients, testing the samples collected from patients, or calculating the results, knows which participants received the vaccine and which did not (this is called a “double-blind” study). Researchers are also studying how long the vaccine can be used before it expires, taking into consideration how it will be transported and stored.
- Regulatory review and approval: Scientists with the FDA and CDC closely review the data from the clinical trials before a vaccine can be licensed and approved.
- Additionally, the Advisory Committee on Immunization Practices (ACIP) – a group of independent medical and public health experts who review data on new and existing vaccines and diseases – will make recommendations for approval and use within specific age groups.
- Manufacturing: The vaccine goes into production. The FDA inspects the factory and approves drug labels.
- Quality control: Scientists and government agencies use databases such as the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink Project to monitor vaccine safety.
- VAERS collects and analyzes reports of adverse events that happen after vaccination. Anyone can submit a report, including parents, patients, and health care professionals. That report is then evaluated by medical experts and examined for trends to identify any vaccine safety issues.
- The Vaccine Safety Datalink Project, a network of health care organizations across the U.S., analyzes health care information from over 24 million people, which scientists use to actively monitor safety.
- Vaccine recommendations may change if safety monitoring reveals new information on vaccine risks (like if scientists detect a new serious side effect).
- The approved COVID-19 vaccines will be utilizing these standard safety programs, which are already in place, and will also be utilizing the new quality control program known as V-Safe. This new program is a vaccination health checker which uses smartphone technology to monitor and receive reports about adverse side effects.
Sources:
CDC: Ensuring the Safety of COVID-19 Vaccines in the United States
CDC: Ensuring COVID-19 Vaccines Work
Children’s Hospital of Philadelphia: Questions and Answers about COVID-19 Vaccines
CDC: V-safe After Vaccination Health Checker
